All about magnesium chloride (MgCl2)
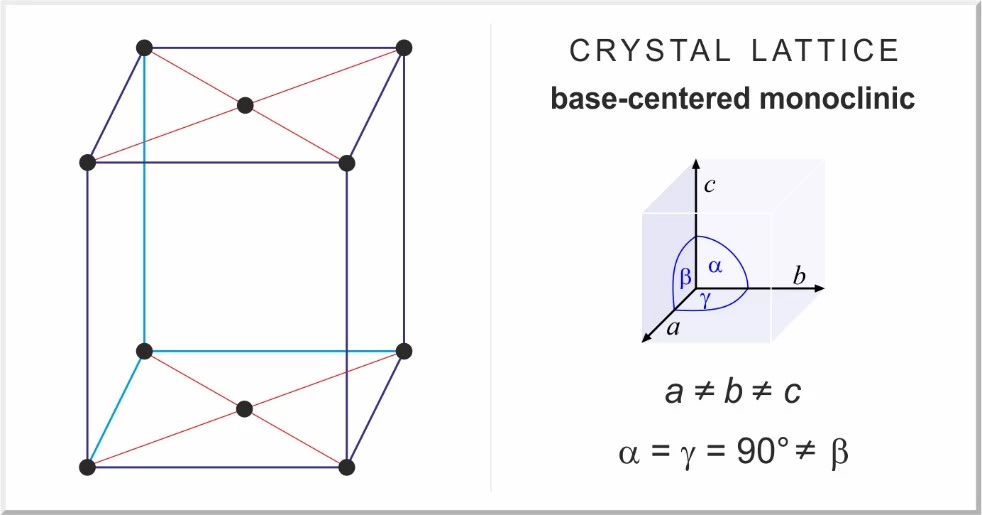
Monoclinic crystal form corresponding to MgCl2.6H2O
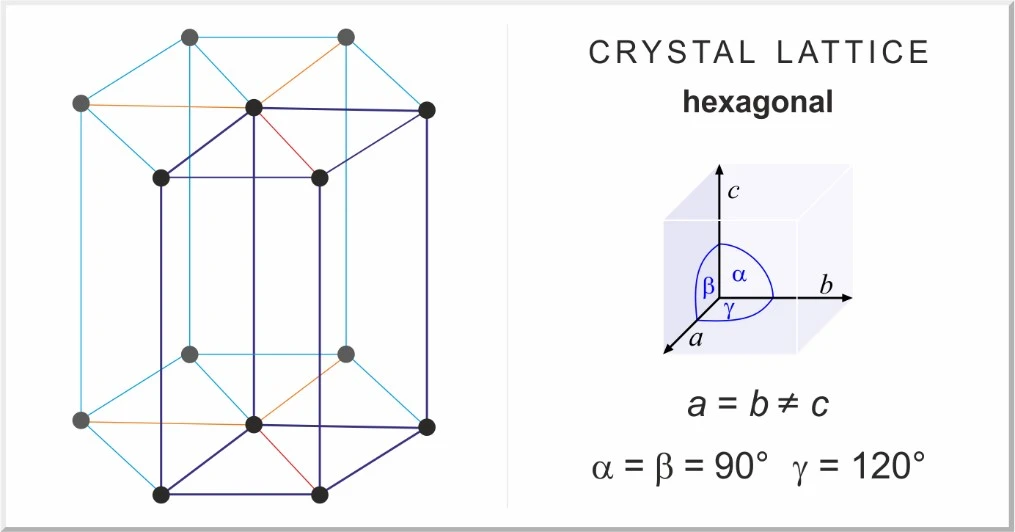
The hexagonal crystal form of MgCl2
Magnesium chloride is an inorganic metal halide that is colorless, odorless and soluble in water. The crystalline form of magnesium chloride 6 is monoclinic, and the crystalline form of anhydrous magnesium chloride is hexagonal. Magnesium chloride is known in the industry as chlorine water.
Magnesium chloride is naturally found in the solid phase in Carnallite rock with the molecular formula KCl, MgCl2.H2O, and it is found in the liquid phase in brines. Hydrated magnesium chloride has a different number of waters in the form of MgCl2.nH2O n=1, 2, 4, 6, 8.
Some properties of magnesium chloride
Preparation of magnesium chloride
The preparation of magnesium chloride is either through the purification of brines or through the reaction of magnesite ore with hydrochloric acid. Of course, magnesium chloride can also be a by-product in potassium extraction.
Its water loss mechanisms are as follows:
1) MgCl2.6H2O→MgCl2.4H2O+2H2O
2.1) MgCl2.4H2O→MgCl2.4H2O+2H2O
2.2) MgCl2.4H2O→MgOHCl+HCl+3H2O
3.1) MgCl2.2H2O→MgCl2.H2O+H2O
3.2) MgCl2.2H2O→MgOHCl+H2O+HCl
4.1) MgCl2.H2O→MgCl2+H2O
4.2) MgCl2.H2O→MgOHCl+HCl
As you can see, for the preparation of anhydrous magnesium chloride, it is not possible to reach the anhydrous form simply by heating. Ammonium chloride should be added to it in order to achieve the anhydrous form and to prevent magnesium chloride from degrading and turning into magnesium oxychloride and hydrochloric acid during heating. In this case, it prevents the reactions of 2-2, 2-3 and 2-4.
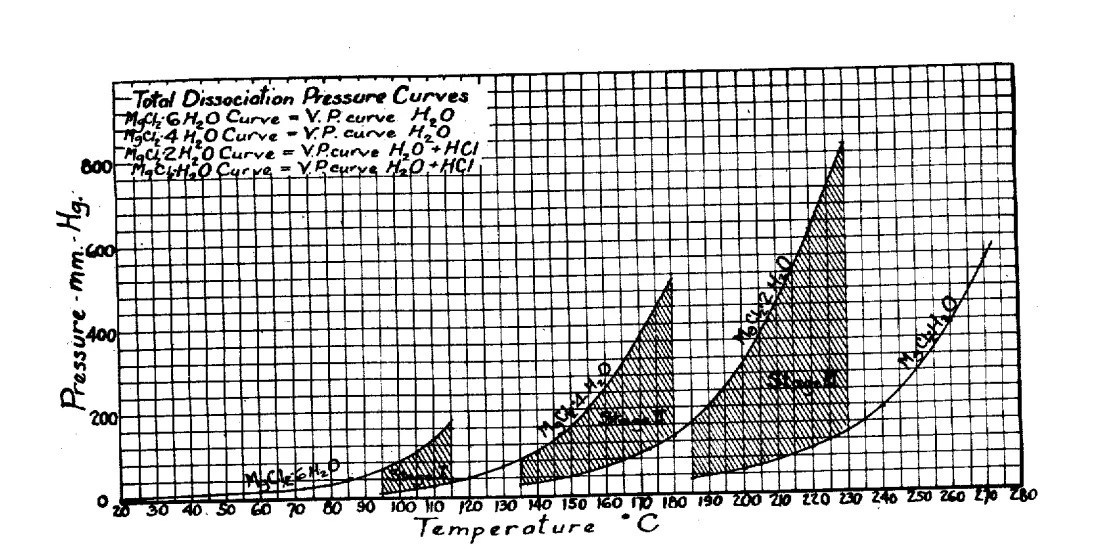
The figure below is the diagram of different phases of magnesium chloride in different forms of hydridation.
All magnesium hydrates are moisture absorbent. You can see the solubility of these salts in the figure below:
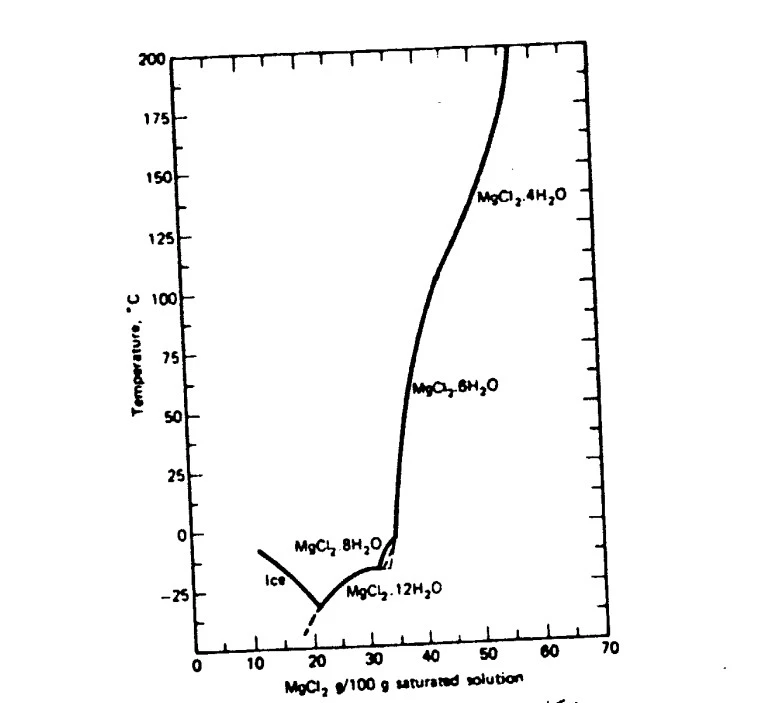
The stable form of aqueous magnesium chloride at 0-100°C is magnesium chloride hexahydrate, also known as Bischofite. In this case, magnesium chloride is connected with 6 water molecules through hydrogen bonding.
Conclusion:
In this article, we tried to learn about the properties of magnesium chloride. In future articles, we will examine the properties of this valuable product. Shimi Gostar Aran Company is the producer of this product; if you want to buy this product, contact our experts.
References:
- Li WK, Zhou GD, Mak T. Advanced structural inorganic chemistry. OUP Oxford; 2008 Mar 27
- Haynes WM. CRC handbook of chemistry and physics. CRC press; 2016 Jun 24.